7. What do living systems use to control shifts in pH and why do they work?
Abstruse
A fundamental variable in civilization medium is its pH, which must be controlled by an appropriately formulated buffering regime, since biological processes are exquisitely sensitive to acrid–base chemistry. Although awareness of the importance of pH is fostered early in the training of researchers, in that location are no consensus guidelines for best practice in managing pH in cell cultures, and reporting standards relating to pH are typically inadequate. Furthermore, many laboratories adopt bespoke approaches to controlling pH, some of which inadvertently produce artefacts that increase dissonance, compromise reproducibility or lead to the misinterpretation of data. Here, we employ real-time measurements of medium pH and intracellular pH under live-cell culture weather condition to describe the effects of diverse buffering regimes, including physiological CO2/HCOthree − and non-volatile buffers (e.g. HEPES). We highlight those cases that outcome in poor control, non-intuitive outcomes and erroneous inferences. To improve data reproducibility, we advise guidelines for decision-making pH in culture systems.
Introduction
Biomedical laboratories routinely perform cell culture to produce a cellular environment that is precisely defined, well controlled and physiologically relevant. Among the main chemical variables of culture systems is the concentration of H+ ions, oftentimes referred to equally protons. These ions are present in every aqueous compartment, not least from the ionization of h2o. Various solutes can go protonated, thereby establishing multiple chemical equilibria involving H+ ions. Consequently, the concentration of complimentary H+ ions is not intuitive to predict, merely fortuitously uncomplicated to mensurate (eastward.g. with electrodes or indicator dyes). For over a century, the pH calibration has been the reporting standard for the concentration of free H+ ions1, that is, the form that is able to protonate targets and mail service-translationally change proteins, such as enzymes or receptors2,iii,four. The much larger pool of buffered H+ ions can, however, influence pH through dynamic re-equilibration5.
In that location is still a widely held misconception that buffers have an inherent power to set the pH of a solution to a pre-defined level. More than accurately, in a system of one ascendant buffer, pH is related to the concentration of the buffer's protonated (HB) and unprotonated (B) forms, and the acid dissociation constant (pChiliad a):
$${\mathrm{pH}} = {\mathrm{p}}K_{\mathrm{a}} + {\mathrm{log}}\frac{{\left[ {\mathrm{B}} \right]}}{{\left[ {{\mathrm{HB}}} \correct]}}.$$
(1)
Consider the dissolution of HEPES buffer (4-(ii-hydroxyethyl)-1-piperazineethanesulfonic acid), typically supplied as a pulverisation of the free acid form. This produces an acidic solution that must be titrated (with base, e.g. NaOH) to the desired pH; once the [B]/[HB] ratio is raised to the required level, pH will remain stable, unless there is an additional source of acid or base. In live-prison cell culture, pH disturbances are an inescapable consequence of metabolism and there is a general tendency for media to undergo acidification, the extent of which is also a function of medium buffering capacity. In a closed arrangement, it can be derived mathematically (meet Supplementary Note) and shown empirically5 that peak buffering capacity is attained when the buffer's protonated and unprotonated forms are equimolar, that is, when medium pH aligns with the buffer's pK a. Many exogenous buffers are available, roofing a wide pH range, including HEPES (p1000 a = 7.3; 37 °C), PIPES (piperazine-N,Northward′-bis(2-ethanesulphonic acid); pG a = half-dozen.7) and MES (2-(N-morpholino)-ethanesulfonic acid; pThousand a = vi.0)6.
A more than agile means of maintaining high buffering capacity involves the regulation of [HB] and [B], so that their ratio is kept at an optimum5. This strategy underpins the reason why circuitous organisms rely on COtwo/HCO3 − buffer (despite low p1000 a = 6.15)7, and have evolved gas exchange surfaces (east.g. lungs) and ion ship epithelia (e.1000. kidneys) to empower CO2 and HCO3 − homeostasis8. The combination of CO2 (an acidic gas) with HCO3 − (a base) produces quantitatively the most important buffer in extracellular body fluids. In civilisation systems, this so-chosen carbonic buffer is stabilized past adding an amount of HCOthree − salt to media and enriching the incubator's atmosphere with CO2. Hither, we chronicle HCOiii − and COii with pH, show how the organisation responds to changes in its components and demonstrate how the equilibrium is afflicted past non-volatile buffers (NVBs) added to augment buffering capacity. Furthermore, we explain how certain cell culture manoeuvres may atomic number 82 to poor pH command. While a number of high-profile guidelines relating to cell civilisation have been published recentlyix,10,xi,12,13, they exercise not comprehensively cover the aforementioned issues pertaining to pH. Based on our observations, we propose guidelines for good exercise in decision-making pH in culture systems.
Results
Monitoring civilization medium pH nether incubation
Buffers are included in civilisation media to control acidity, yet the ensuing pH is non routinely monitored. This becomes a quality control issue whenever the components of buffering are beingness disturbed: for example, in response to metabolic acrid production, or equally a consequence of transferring media between atmospheres of different CO2 fractional pressures (pCO2). The dye Phenol Red (PhR) is routinely included in the media to permit investigators to analysis medium acidity14,15. Such assessment could exist washed 'by eye', but a more than quantitative readout of pH is obtainable from the PhR absorbance spectrum, which can be recorded on plate-reader platforms with an incubator bedchamber (e.yard. Cytation 5, BioTek). To obtain a scale curve, freshly prepared standards of known pH were scanned in a COii-free atmosphere to prevent the acidifying effect of CO2. Calibration solutions had no added HCO3 −, equally otherwise this basic substance would have reacted slowly with H+ ions and then escape as CO2 gas. Fig. 1a shows absorbance spectra of PhR in bicarbonate-free Dulbecco'south modified Eagle's medium (DMEM) (D7777, Sigma-Aldrich) supplemented with 10% foetal bovine serum (FBS) and i% penicillin–streptomycin (PS; 100 U mL−one penicillin, 0.1 mg mL−one streptomycin), 10 mM HEPES and 10 mM MES (2-(Northward-morpholino)-ethanesulfonic acid), and titrated to a target pH with NaOH. To correct for pH-contained variables, such as low-cal path or PhR concentration, absorbance was sampled at two wavelengths that reply differently to pH. Good resolving ability is attained by rationing absorbance at 560 and 430 nm (Fig. 1b). The best-fit equation can so be used to convert PhR ratio assayed in subsequent experiments to pH.
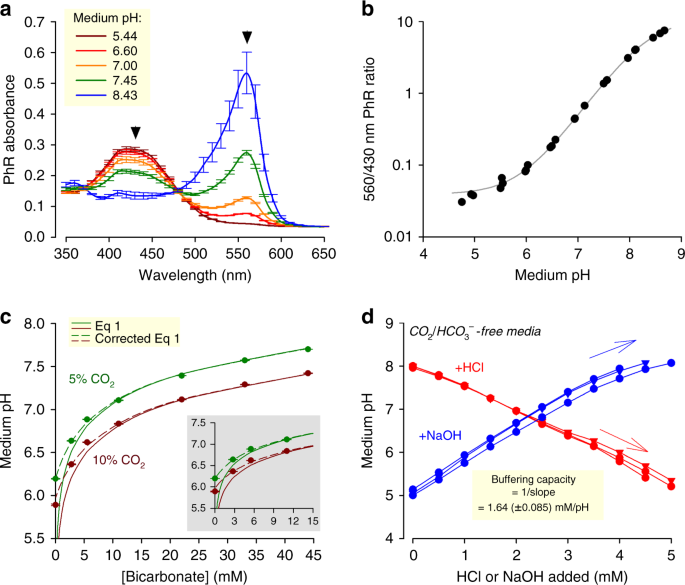
Measuring and setting medium pH under incubation. a Absorbance spectrum of Phenol Carmine (PhR) in Dulbecco's modified Eagle's medium (DMEM) (D7777) with ten% foetal bovine serum (FBS), 1% penicillin–streptomycin (PS), 10 mM HEPES (4-(two-hydroxyethyl)-1-piperazineethanesulfonic acrid) plus ten mM two-(N-morpholino)-ethanesulfonic acrid (MES), and titrated (5 M HCl or 4 G NaOH) to the indicated pH. Arrows indicate wavelengths for optimal ratiometric analysis. b pH dependence of 560/430 nm ratio, fitted to curve: pH = eight.35 + log((10.9 – ratio))/(ratio – 0.0392)). c Controlling equilibrium pH past varying pCO2 and [HCOthree −] in DMEM (D7777) supplemented with 10% FBS and 1% PS. Dashed line plots Eq. 1. Continuous line is best fit to Eq. 3 (corrected version of Eq. ane), which accounts for buffering past serum (best fit: one.eleven mM pH−1). Inset replots the data at low [HCO3]. d Empirical determination of intrinsic buffering chapters of DMEM (D7777; ten% FBS/1% PS, 25 mM glucose) nominally lacking buffers; titration with either HCl or NaOH. Changed of slope provides an judge of buffering due to serum proteins and media salts. All measurements were repeated three times (3 technical replicates each). Data are shown as mean ± SEM
Setting medium pH by pCO2 and [HCO3 −]
In principle, it is possible to control pH with one of many commercially bachelor buffers, simply the almost physiologically relevant one is CO2/HCOiii −. Incubators maintain a CO2-rich atmosphere (typically five%) to enable COii/HCOiii − buffering. A salt of HCOthree − must be included in the medium to balance the spontaneous H+-yielding CO2 hydration reaction, and stabilize pH:
$${\mathrm{CO}}_{\mathrm{2}}\left( {{\mathrm{gas}}} \right)\rightleftarrows{\mathrm{HCO}}_{\mathrm{three}}^{\mathrm{ - }}\left( {{\mathrm{aq}}} \right){\mathrm{ + H}}^{\mathrm{ + }}\left( {{\mathrm{aq}}} \right).$$
(two)
Conveniently, pH can be titrated in the range between ~vi and ~eight by varying the concentration of HCOthree −. Figure 1c plots the relationship between pCO2, [HCO3 −] and pH measured in DMEM (D7777, Sigma-Aldrich) containing 10% FBS and 1% PS. To go on osmolality constant, any reduction in NaHCOthree was matched by a compensatory rise in NaCl. Over the alkaline range, pH reported by PhR is in very close agreement with the prediction of the Henderson–Hasselbalch equation (Eq. 1), consequent with COtwo/HCO3 − beingness the dominant buffer (pChiliad a = 6.15; COii solubility 0.024 One thousand atm−i, i.due east. 1.2 mM in v%)7. Notwithstanding, at low [HCO3 −], Eq. ane underestimates pH. This discrepancy arises because the so-called 'intrinsic' buffers in medium (such every bit proteins included in serum) react with H+ ions generated past COii hydration, pushing the equilibrium (Eq 2) towards a college [HCOiii −] and lower [H+], that is, a less acidic medium. This correction is derived mathematically in the Supplementary Annotation. Thus, the concentration of HCO3 − required for attaining a target pH is:
$$\left[ {{\mathrm{HCO}}_3^ - } \right] = \left[ {{\mathrm{CO}}_2} \right] \times x^{{\mathrm{pH}}_{{\mathrm{target}}} - six.15} + \beta _{\mathrm{intrinsic}} \times \left( {{\mathrm{pH}}_{{\mathrm{target}}} - 7.4} \right).$$
(3)
By best plumbing fixtures the data to this equation, intrinsic buffering (β intrinsic) was estimated to be 1.1 mM pH−1. Alternatively,β intrinsic can be measured empirically from the response of pH to the footstep-wise addition of acid or base (1.half-dozen mM pH−1; Fig. 1d).
Stability of CO2/HCO3 − buffering
In most instances, media are prepared to a neutral or alkaline pH, and over this pH range, the Henderson–Hasselbalch equation (1) is acceptable for predicting equilibrium pH. However, the robustness of Eq. 1 depends on the accuracy of pCO2 and [HCO3 −] measurements. In many instances, information technology may be appropriate to assume that the amount of HCO3 − salt added to a medium accurately predicts the concluding concentration of base of operations; however, some formulations contain weak acids that react with HCO3 − salts. Nether these circumstances, Eq. i will underestimate pH, and therefore straight pH measurements are advocated. For example, the add-on of 22 mM of NaHCO3 to media supplemented with lactic acid will not produce the expected pH of 7.4 due to the titration reaction (Fig. 2a). If, instead, media contained a table salt of lactic acid (e.g. Na-lactate), so the acid-titration reaction with HCO3 − volition not take place, and Eq. 1 adequately approximates pH (Fig. 2a). The difference in behaviour between lactic acid and its cohabit base can be explained in terms of equilibria:
$${\mathrm{Lactic}}\,{\mathrm{acid\rightleftarrows Lactate + H}}^{\mathrm{ + }}.$$
Around neutral pH, this equilibrium is shifted far to the correct. After dissolving a lactate common salt, just a tiny fraction of lactate volition protonate, thus the change in pH is negligible. In contrast, lactic acid added to a medium undergoes near-complete deprotonation, which reduces pH.
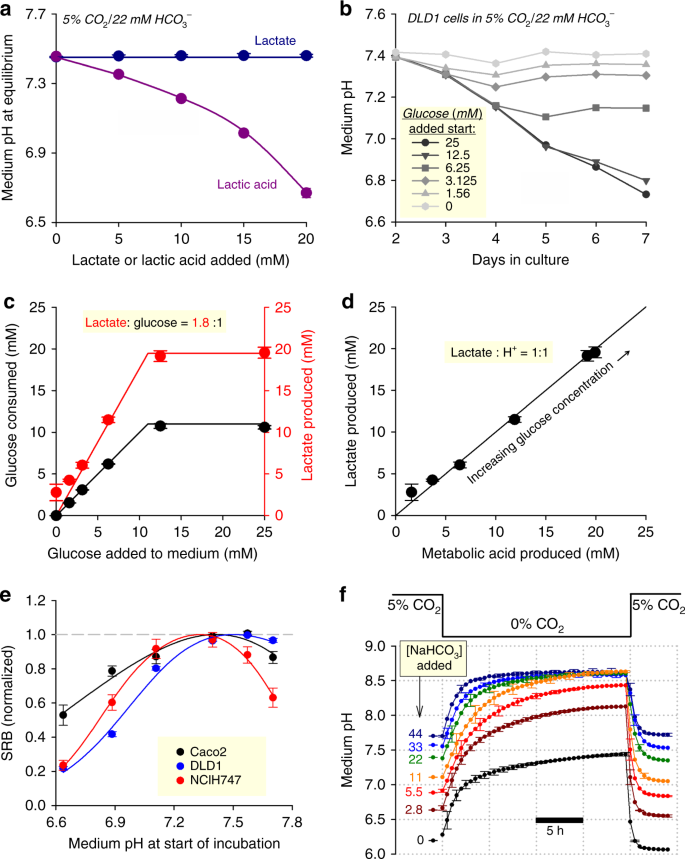
The control and stability of pH in CO2/HCO3 −-buffered medium. a Outcome of increasing [lactic acid] or [lactate] on equilibrium pH of medium (DMEM D5030) with 22 mM NaHCO3 (10% foetal bovine serum (FBS), 1% penicillin–streptomycin (PS)) placed in 5% CO2. b Outcome of metabolic lactic acid product by DLD1 cells (seeding density 4,000 cells per well, growth area 0.32 cmii per well) on the pH of medium (DMEM D5030; 10% FBS, ane% PS) containing 0–25 mM glucose (osmotically compensated with NaCl). Error bars omitted for clarity. c Effect of varying starting glucose concentration on net glucose uptake and lactate production probed on the seventh solar day of incubation. xc% of glucose is metabolized to lactate. d Relationship between lactate production (measured past biochemical assay) and total acid production (calculated from the pH change and buffering capacity). Slope of 1.0 indicates that medium acidification is due to lactic acid product. e Jail cell growth of iii colorectal cancer jail cell lines (seeding density 4,000 cells per well, growth area 0.32 cm2 per well) measured from protein biomass (sulforhodamine B (SRB) assay) after 6 days of incubation in DMEM (D7777; ten% FBS, 1% PS, 25 mM glucose) over a range of starting pH attained past varying [HCO3 −] at abiding 5% CO2. Data are normalized to the optimum pH derived by best fit to biphasic bend. Optimal growth is near the physiological pH of vii.4. f Effect of varying pCO2 on medium pH, mimicking the withdrawal of medium from nether COii incubation. All experiments were repeated three times (three technical replicates each). Data are shown as mean ± SEM
Physiologically, a primary source of lactic acid is glycolytic metabolism (~1:one lactate:H+ stoichiometry16), which inadvertently reduces the pH of a finite volume of medium by reacting with its HCO3 − ions. An exemplar time course of medium acidification produced past DLD1 cells is shown in Fig. 2b for a range of starting glucose concentrations. Below a starting glucose concentration of ~12 mM, substrate availability is rate-limiting for lactic acid output, measured from lactate accumulation and glucose consumption afterwards half dozen days of incubation (Fig. 2c). When glucose availability was not charge per unit-limiting (>12 mM), DLD1 cells were able to produce ~xx mM of lactic acrid over a period of 6 days. The extent to which lactic acid production underpins medium acidification was determined by comparing lactate measurements with cumulative acid production:
$${\mathrm{Acid}}\,{\mathrm{produced}} = - \mathop {\sum }\nolimits( \beta \cdot \Delta {\mathrm{pH}}).$$
Here, buffering chapters (β) is the sum of intrinsic buffering and CO2/HCOiii −-dependent buffering (come across Supplementary Notation). Plotting the relationship between these two contained measurements (Fig. 2d) demonstrates that in DLD1 cells, medium acidification is entirely deemed for past glycolytic lactic acid product.
Considering the magnitude of lactic acid product, medium [HCO3 −] volition invariably fall below starting levels during extended periods of incubation. As a pre-emptive mensurate, many types of media are formulated to contain 44 mM NaHCO3, an backlog to provide a safety margin for adequate base. All the same, at five% CO2, such media will equilibrate at pH 7.7, which is a supra-physiological level that can accept untoward furnishings on cellshalf-dozen. To demonstrate the importance of physiological [HCOiii −], cell growth was studied at various levels of pH attained by varying [HCO3 −], over a catamenia of vi days in serum-containing medium. Cellular growth, in the presence of 10% FBS and i% PS, was interrogated by a cytotoxicity assay based on the protein-bounden probe sulforhodamine B (SRB)17. In three colorectal cancer cell lines (NCI-H747, DLD1, Caco2), growth was optimal near pH vii.4, and the effect of incubation at pH 7.7 varied, with the strongest inhibition of growth in NCI-H747 cells (Fig. 2e). The notion of an optimal pH for cell growth has been noted past others18, merely the molecular mechanism behind this response is not well defined. Reduced proliferation at pH >seven.4 may, for case, chronicle to excessive debinding of H+ ions from sensors and 'ionic trapping' of lactate in alkaline cytoplasm.
Another factor that may contribute towards pH instability relates to pCO2. In vivo, most mammalian cells will be exposed to a tightly regulated pCO2, which helps maintain pH homeostasis. By illustration, feedback circuits in incubators are designed to keep pCOii abiding. This environmental constancy is, however, not always possible with cultured cells, as various protocol steps may require transfers between atmospheres of different pCOtwo (eastward.g. in and out of an incubator). There are 2 implications of this. Showtime, a medium that had been titrated in a CO2-free temper (due east.grand. at the demote) will acidify upon placement in a COtwo incubator6. Second, data collected from cells that had been withdrawn from an incubator may be influenced by the sharp rise in pH6. This issue could exist addressed by minimizing COii loss from the medium (due east.g. past enclosing the civilisation dish in a regulated temper), or past superfusing with solutions pre-equilibrated with 5% CO2. The loss of CO2 from a medium can be tracked using the time course of alkalinization evoked when HCO3 −-containing media equilibrated at 5% CO2 is transferred into a COii-costless atmosphere (Fig. 2f). The small volume of media contained in 96-well plates begin to alkalinize immediately, with a time abiding of two–3 h. The reverse reaction has a time-constant of 45 min, indicating that freshly prepared media may crave an hour to equilibrate inside a COtwo incubator.
Effect of NVBs on the stability of medium pH
The perceived drawbacks of COii/HCOthree −, namely its volatility and weaker buffering at depression pH, accept led to the use of exogenous NVBs such as HEPES, PIPES and MES19. It is crucial that the preparation of media containing such buffers carefully considers the CO2/HCO3 − equilibrium, which takes place under COii incubation. NVB-buffered medium titrated 'at the bench' to a target pH will invariably become more acidic upon placement in a 5% CO2 incubator. For example, bicarbonate-gratis DMEM buffered with xx mM HEPES (a widely used formulation) acidifies by over one-half a pH unit of measurement upon exposure to 5% CO2 (Fig. 3a). Acidification was less pronounced at low pH because the concentration of HCOthree − required to encounter the equilibrium condition is lower. The extent of CO2 hydration could be curtailed by supplementing media with HCO3 − salts. This, yet, produces a two-buffer organisation in which pH dynamics are less intuitive to predict. To demonstrate this instability, media were prepared with 22 mM NaHCO3 and ane of either HEPES, PIPES or MES. These mixtures were titrated 'at the bench' to near the pK a of the elective NVB, and and then promptly placed in a 5% CO2 incubator for continuous pH monitoring. Media prepared this mode demonstrated a substantial caste of pH instability (Fig. 3b). The direction of pH drift is determined by 2 opposing chemical reactions (Fig. 3c): (i) the acidifying outcome of atmospheric COtwo dissolving and reacting with the medium; and (ii) the alkalinizing result arising from the equilibration between HCO3 − ions and the NVB, a wearisome process that had started prior to incubation in 5% CO2. The residue betwixt these opposing processes depends on the starting pH, and produces an assortment of responses that are not intuitive to predict (Fig. 3b).
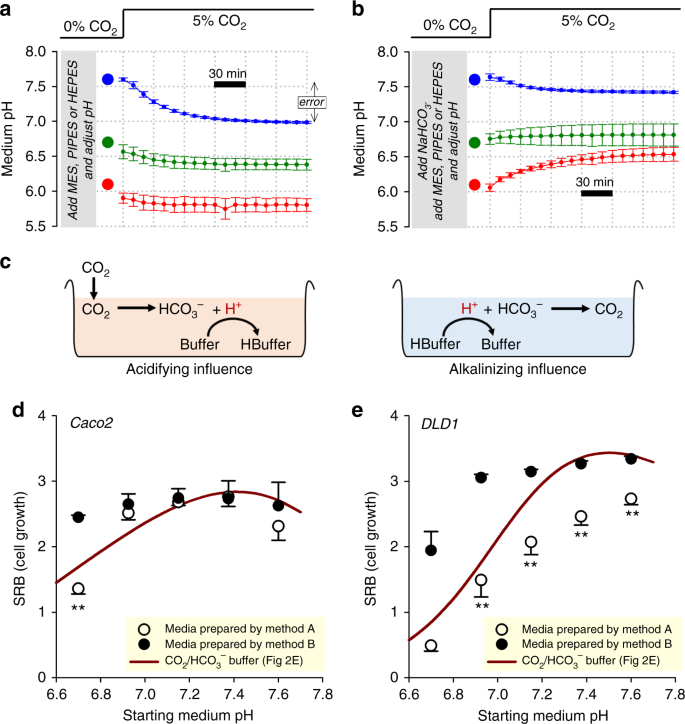
pH dynamics in media prepared with non-volatile buffers without consideration of the CO2-HCO3 − equilibrium. a Medium (DMEM D7777) supplemented with non-volatile buffer (HEPES, PIPES or MES; 20 mM), 10% FBS, ane% PS, and titrated to indicated target pH (large circles). Time courses show pH dynamics (measured from PhR ratio) evoked past placing media inside 5% CO2 incubator, showing a tendency to acidify. b Medium (DMEM D7777) supplemented with 22 mM NaHCO3 plus non-volatile buffer (HEPES, PIPES or MES; 20 mM), and titrated to indicated pH before placement in v% CO2. Fourth dimension courses show pH dynamics evoked by placing media in v% COii incubator, demonstrating pH instability. c Schematic of the chemic processes that underpin medium pH drifts. d pH-dependence of Caco2 cell growth (seeding density 4000 cells per well, growth surface area 0.32 cm2 per well) measured from protein biomass (SRB assay) later vi days of incubation in D7777 (25 mM glucose). Media were prepared by method A (xx mM HEPES/PIPES) or method B (twenty mM HEPES/PIPES plus 22 mM NaHCO3), and placed in 5% COii. To obtain a range of pH values, HEPES- and PIPES-buffered media were mixed in various ratios. Data are plotted as a function of assumed pH (titrated 'at the bench'). Results compared confronting curve obtained with CO2/HCO3 − buffer, plotted against measured equilibrium pH (data from Fig. 2e). due east Experiment performed on DLD1 cells, a more than pH-sensitive line. Data are shown equally hateful ± SD (a, b) or mean ± SEM (d,e). All measurements were repeated three times (three technical replicates each). Statistical tests: two-sided t test (**P < 0.01)
Poorly controlled pH, such as in the instances described higher up, will impinge on the accuracy and reliability of biological recordings. To illustrate this trouble, the pH dependence of growth nether five% COii incubation was measured in NVB-buffered media prepared either with or without the addition of 22 mM NaHCOthree, co-ordinate to the schemes shown in Fig. 3a, b, respectively. Experiments were performed on Caco2 (Fig. 3d) and DLD1 cells (Fig. 3e), representing a weakly and strongly pH-sensitive line, respectively (see Fig. 2e). Growth, measured by the SRB assay after vi days of civilisation, was plotted confronting the pH to which media were titrated 'at the bench' (i.e. the assumed pH). In line with previous studiesxx, PIPES was selected for acidic media and HEPES was chosen for alkaline media; to obtain a range of pH, these media were mixed in various ratios. Every bit a control, growth was measured separately in media prepared with CO2/HCO3 − in a style that produces a predictable starting pH (Fig. 1c). If the error associated with pH instability nether COtwo incubation were negligible, so the pH dependence of growth measured in NVB-buffered media would be the same, irrespective of the method of medium preparation. However, the measured pH–growth human relationship was apparently unlike, depending on how the medium was prepared. NVB-buffered media prepared without NaHCO3 (Fig. 3a; method A) yielded an apparently steeper pH dependence of growth, compared to NVB-buffered media prepared with NaHCO3 (method B). This disparity, which was more pronounced in the strongly pH-sensitive DLD1 line, cannot exist explained merely by the chemical presence of NVBs because both methods used matching concentrations of HEPES and/or PIPES. Also, the differences do not relate to inadequate [HCO3 −] (e.yard. for supplying pH-regulating proteins) considering all media within CO2 incubators eventually accumulate HCO3 − from the spontaneous hydration of COtwo. Instead, the apparent shifts in pH–growth human relationship relate to the pH mistake incurred during medium preparation. When placed inside a COtwo incubator, NVB-buffered media prepared co-ordinate to method A (Fig. 3a) will undergo an acid-shift beyond the pH range. This has the issue of over-estimating the degree of growth inhibition at low pH. In dissimilarity, the pH of NVB-buffered media prepared with NaHCO3 and titrated 'at the bench' (Fig. 3b) will converge towards pH ~7 during CO2 incubation. This produces evidently pH-insensitive growth, because the test range of pH is, in reality, narrowed. Ultimately, the error was introduced because medium pH was set in a manner that did not take into account the CO2-HCOthree − equilibrium.
Notwithstanding the problems described to a higher place, in that location may be valid reasons to supplement media with NVBs (e.g. to limit medium acidification under long-term cell culture of highly glycolytic cell lines)20. The apparent instability of systems containing a NVB plus COtwo/HCO3 − (described in Fig. three) could be addressed past modifying the protocol for preparing media. In the first step, the NVB should be added to bicarbonate-free media, then titrated to a target level 'at the bench'. To include COtwo/HCO3 − buffer, its components must exist added at a concentration ratio that volition exist in equilibrium with the target pH (Fig. 1c). In the case of HEPES-buffered media titrated to pH seven.four, this would require the improver of 22 mM of NaHCOthree and placement in v% CO2. Media prepared this mode will equilibrate to the target pH inside ii h when aliquoted into 96-well plates (Fig. 4a). Note that the equilibration takes longer than in the experiment shown in Fig. 2f because of the resistive action of NVBs towards pH changes. Hence, information technology is possible to combine an exogenous buffer with physiological COii/HCO3 − to improve overall buffering capacity, and even so attain a anticipated pH (Fig. 4b).
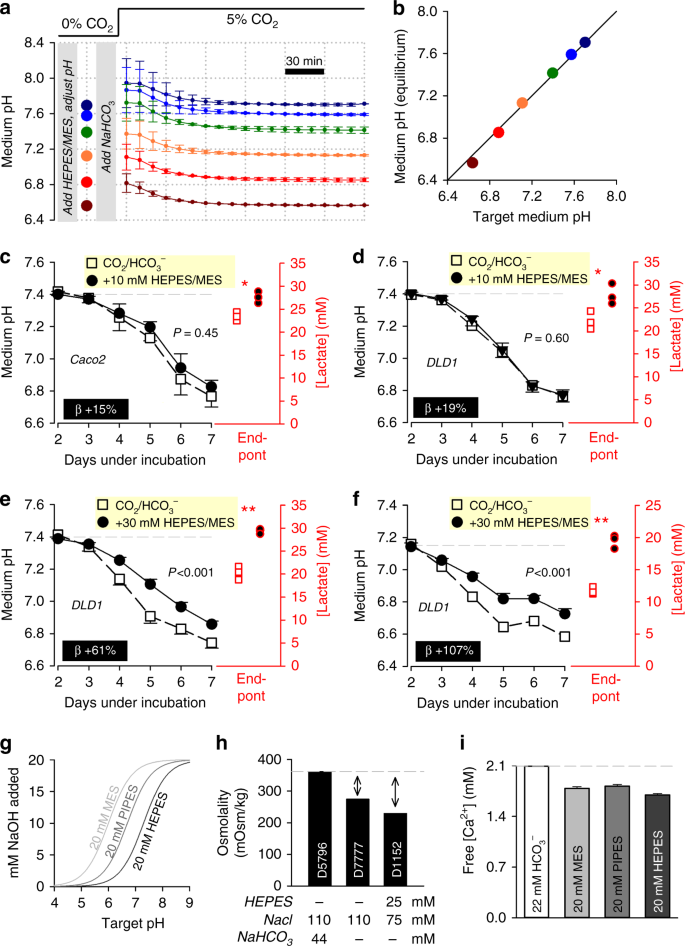
Enhancing buffering capacity of CO2/HCO3 −-containing media with non-volatile buffers, with consideration of the CO2-HCOiii − equilibrium. a Medium (DMEMD 7777, 10% FBS, ane% PS, 25 mM glucose) supplemented with non-volatile buffer HEPES and MES (10 mM), and titrated to indicated target pH (big circles). NaHCOthree and so added to a concentration expected to exist in equilibrium with five% CO2 at target pH (Fig. 1c). Fourth dimension class of pH equilibration under five% CO2 from different starting levels. Repeated iii times (three technical replicates each). b Proficient agreement between target and measured equilibrium pH. c Time course of medium acidification in Caco2 cells (seeding density four,000 cells per well, growth surface area of 0.32 cm2 per well). Media buffered with v% CO2/22 mM HCOiii −, or the combination of CO2/HCO3 − plus 10 mM HEPES/MES (a 19% increase in time-averaged buffering, β). Medium lactate accumulation at the end point was greater with enhanced buffering. d Experiment performed on DLD1 cells. due east Experiment performed on DLD1 cells with 30 mM HEPES/MES. f Experiment repeated from a more acidic starting pH, at which 30 mM HEPES/MES is expected to provide one-half of total buffering. Measurements repeated four times (three technical replicates each). Statistical tests: lactate measurements tested by two-sided t test (*P < 0.05, **P < 0.01); fourth dimension courses tested past 2-way assay of variance (ANOVA) (P value for the effect of buffering is stated). yard Increasing buffering chapters with non-volatile buffers likewise increases osmolarity due to the buffer molecules and the base of operations required for titration. Calculated [NaOH] required to titrate MES, PIPES or HEPES buffer to a target pH. h Osmolality of three different media formulations. Arrows evidence gap in osmolality in HCOthree −-free media, which can be filled with buffer and acid–base of operations required for titration, plus additional NaCl required to bring osmolality to a physiological level. Notation, in the case of medium D7777 and D1152, a total of 88 and 132 mOsm kg−ane tin be added, respectively. i Free [Caii+] measured past electrode, showing partial Ca2+ chelation by non-volatile buffers. Data are shown every bit hateful ± SEM. Repeated three times
To examination how additional buffering affects the time course of medium acidification, Caco2 or DLD1 cells were cultured in CO2/HCO3 −-buffered DMEM supplemented with 10 mM HEPES and 10 mM MES, prepared every bit described in Fig. 4a. This combination of ii NVBs adds a constant buffering capacity over the pH range 6 to 8, making it easier to place biological effects in media undergoing acidification. Somewhat paradoxically, 10 mM HEPES/MES did not meaningfully reduce medium acidification (Fig. 4c, d), despite the obvious increase in buffering ability. Notwithstanding, the enhanced buffering was found to increase lactate production, implying a greater collective glycolytic rate. The resulting pH fourth dimension course was unaffected, because the augmented buffering capacity was cancelled out by the stimulated metabolic acid production, which can be expressed mathematically equally:
$${\mathrm{Change}}\,{\mathrm{in}}\,{\mathrm{medium}}\,{\mathrm{pH}} = - \frac{{{\mathrm{lactic}}\,{\mathrm{acid}}\,{\mathrm{production}}}}{{{\mathrm{buffering}}\,{\mathrm{capacity}}}}.$$
These observations tin can be explained in terms of the inhibitory feedback of acerbity on metabolic rate21,22,23. Augmented buffering reduces the degree of medium acidification, which is permissive for a higher metabolic charge per unit. As expected from a simple pH-operated feedback excursion, the ensuing pH time course volition follow an unchanged trajectory. When the experiment was repeated on DLD1 cells using a much college (xxx mM) concentration of HEPES/MES, lactate production was still stimulated, but non to a degree that would starting time the increase in buffering (Fig. 4e). The furnishings of 30 mM HEPES/MES become more axiomatic when starting pH is reduced (i.e. when COii/HCO3 − buffering is weaker) (Fig. 4f). Thus, information technology is possible to curtail medium acidification with thirty mM HEPES/MES, but less than anticipated from its buffering chapters per se. Whilst these observations should not be generalized to all cells, they emphasize the importance of making confirmatory measurements of pH, and taking into consideration the biological responses to increased buffering, such as metabolic stimulation.
Two boosted precautions must be taken when using NVBs. The first issue relates to the titration of these buffers with acids (e.g. HCl) or bases (e.m. NaOH), which introduces additional osmolytes (Na+, Cl−) into the medium (Fig. 4g). The build-upward of osmolytes can be substantial, for example, the improver of twenty mM HEPES and titration to pH 7.four, introduces ~30 mOsm kg−1 of additional osmolytes (i.e. backlog of ~10%). A major increase in osmolality would atomic number 82 to cell shrinkage, changes in membrane tension and potentially a myriad of downstream effects24. Indeed, supra-physiological osmolality is probable to influence the results of the experiment shown in Fig. 4e, f. Some media formulations are bachelor without buffers, giving some elbowroom for adding extra osmolytes within physiological limits. For example, a full of 88 mOsm kg−1 of boosted osmolytes can be added to HCOiii −-free medium D7777 (Sigma-Aldrich) for the last solution to attain the same osmolality as ready-to-use medium D5796 (Sigma-Aldrich) (Fig. 4h). The second event to consider relates to the binding properties of buffers. Although NVBs are primarily chelators of H+ ions, they also show small affinity for Ca2+ ions. Lower [Ca2+] reduces the driving force for Ca2+ entry into cells and hence the state of Ca2+ signalling cascades25. Solutions containing 20 mM HEPES, PIPES or MES will reduce Ca2+ past ~10–15% (Fig. 4i). Whilst this may not have a paramount event on Ca2+-dependent backdrop, information technology will contribute towards increased dissonance and weaker statistical power. This issue could be avoided by calculation CaClii to compensate for the chelation.
High-throughput assay of intracellular pH
A fundamental reason why changes in medium pH (controlled or unwarranted) can influence cellular physiology is because intracellular pH (pHi) responds to changes in extracellular pH (pHeast). This coupling arises because the proteins that regulate pHi are as well sensitive to pHe, and a re-balancing of transmembrane acid–base fluxes alters steady-state pHi. A major contributor to these acid–base fluxes are HCO3 − transporters, which are agile only in the presence of CO2/HCO3 − buffering8,26. Thus, an assessment of the effects of medium pH and buffering authorities on cell behaviours should consider actions mediated through changes in pHi. Plate-based imaging platforms allow high-throughput fluorescence measurements that can capture the population distribution of pHi in a monolayer. These pHi data tin can be obtained past loading cells with pH-sensitive fluorescence dye cSNARF127. To identify the centroids of cells, nuclei can be stained with Hoechst-33342, which is spectrally resolvable from cSNARF1. After a catamenia of loading (10 min) and wash-out in dye-complimentary media, stacks of images were collected, corresponding to 447 nm fluorescence excited at 377 nm (Hoechst-33342) and of 590 and 640 nm fluorescence excited at 531 nm (cSNARF1) (Fig. 5a). Offline, the pHi in individual cells was inferred from the cSNARF1 fluorescence probed around nuclei, identified past particle assay of Hoechst-33342 images. After ratioing background-subtracted fluorescence at 590 and 640 nm, pHi can be sampled individually for each cell (Fig. 5a). An example of a suitable code, written as a MATLAB script, is included as Supplementary Code 1. This approach was beginning applied to generate a calibration curve with the nigericin method28, in which cells are incubated in solutions containing 100 µM nigericin (a H+/K+ ionophore), 140 mM KCl (to balance intracellular K+), 0.5 mM EGTA, 1 mM MgCl2, 10 mM MES (for pH <vii) or x mM HEPES (for pH >seven) titrated to a desired target pH in a CO2-free atmosphere. The scale curve shown in Fig. 5b was adamant in CO2-free conditions, and the best-fit equation tin can be applied to catechumen measured fluorescence ratio into pH for cells under various test weather.
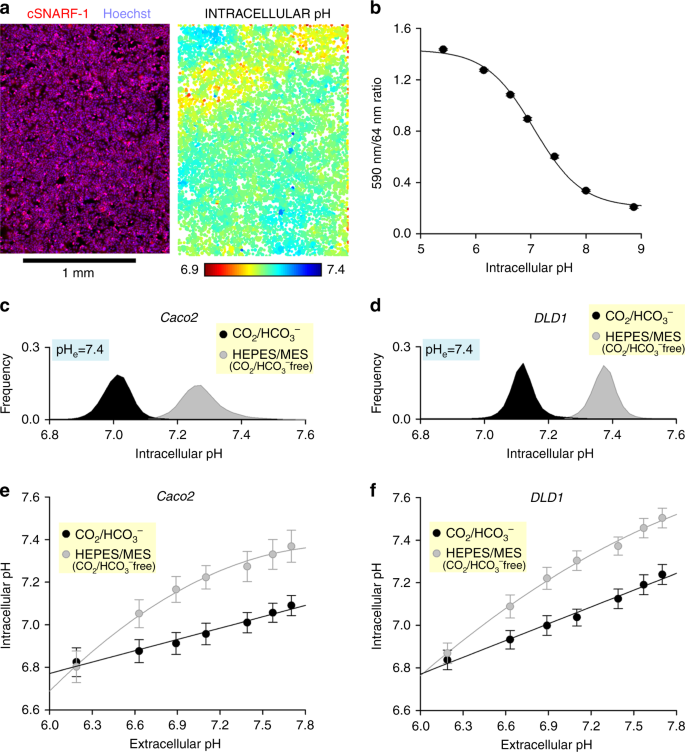
Effects of buffering regime and medium pH on intracellular pH, measured using a high-throughput imaging method. a Monolayer of DLD1 cells imaged with Cytation v plate reader. Image on left shows superimposition of cSNARF1 and Hoechst-33342 fluorescence maps. Image on correct shows pH in individual cells, identified past nuclear staining. b Calibration curve determined from ix colorectal cancer prison cell lines (LS174T, PMFKO14, LS513, HCT15, SW620, GP2D, HCT116, Caco2, RW2892; seeding density 100,000 cells per well, growth area 0.56 cm2 per well). Iii technical replicates each. All-time-fit curve: pH = 6.978 + log((ane.497 −ratio)/(ratio − 0.221)). c Histogram of intracellular pH in Caco2 monolayers bathed in D7777-based media (25 mM glucose) at pH vii.four, buffered past either v% CO2/22 mM HCO3 −, or x mM HEPES + MES titrated to 7.four (COii gratuitous). Note the substantial alkalinization in the absence of physiological buffer. Repeated 3 times (three technical repeats each). d Experiment performed on DLD1 monolayers. e Upshot of medium pH on intracellular pH in Caco2 monolayers. The pH of CO2/HCO3 −-buffered media was varied past changing [HCO3 −] (incubation in 5% CO2). In contrast, the pH of HEPES/MES-buffered media were titrated to target pH with NaOH at the bench (incubation in 0% COtwo). Best fit: linear (CO2/HCO3 −) or polynomial (HEPES/MES). Note that intracellular pH is more than responsive to changes in extracellular pH in the absenteeism of physiological (CO2/HCO3 −) buffer. f Experiment repeated on DLD1 monolayers. Data are shown equally mean ± SD. Repeated three times (three technical repeats each)
This pHi imaging approach was used to investigate the effect of CO2/HCOthree − buffering on pHi. Figure 5c, d show histograms of pHi in populations of Caco2 or DLD1 cells, incubated either in media buffered with 5% CO2/22 mM HCOthree − (equilibrated at pH vii.iv), or CO2/HCOthree −-free media buffered by x mM HEPES and 10 mM MES (titrated to pH 7.4). In the absence of HCOiii − ions, the pHi of DLD1 and Caco2 cells was shifted in the alkaline direction by 0.3 units, which is owing to the inactivation of pHi-regulating HCOiii − transporters. The management and magnitude of this effect is likely to be prison cell blazon-dependent, and therefore not intuitive to predict. Past repeating these experiments over a range of media pH, it is possible to map the pHeast–pHi relationship (Fig. 5e, f). The pHi of cells was more sensitive to changes in pHeast in the absence of CO2/HCO3 −. For example, pHi in Caco2 cells acidified past twice as much in the absence of COtwo/HCO3 − in response to a drop in pHe from 7.4 to half dozen.four (Fig. 5e). Since the majority of H+ targets are intracellular, such buffer regime-dependent changes in pHe–pHi coupling tin can lead to erroneous inferences concerning the mechanisms of jail cell responses to microenvironmental acid–base of operations challenges.
Discussion
Research in virtually every biomedical laboratory relies on jail cell culture, either to maintain cells in a state that is conducive for physiologically relevant activity or to explore the furnishings of controlled chemic, concrete or biological influences. Cultured cells volition remain an essential biological resources, offering a tractable model for characterizing pathways, recording responses and manipulating disease-related processes29. Even so, the translational relevance of findings borne from civilisation systems is critically dependent on the extent to which in vitro conditions relate to in vivo setting. Furthermore, the value of any experimental finding is determined by its reproducibility. However, 70% of scientists surveyed recently by Nature were unable to reproduce another'southward experiment30, and a mail-publication analysis has suggested a reproducibility rate of equally little as 10% in cancer biology31. The inadequate quality of preclinical information has been linked to the loftier failure charge per unit of agents progressing from in vitro validation to stage Three testing, of the order of 95% in cancer research32. 1 factor contributing towards these outcomes has been attributed to variables relating to environmental conditions31.
Commercial sources of media offer a wide range of formulations, summarized in Fig. 6a for three major types: DMEM, MEM and RPMI-1640. Fewer than half of the available formulations contain physiological [HCOthree −], and a substantial number of options include media with considerably lower or higher [HCOiii −], which would produce acidic and alkaline conditions, respectively (Fig. 2a). Special precautions are needed with various formulations supplemented with HEPES because these may produce unexpected pH dynamics inside CO2 incubators (Fig. 3). Formulations that lack COii/HCO3 − and any major NVB provide are useful starting point for producing bespoke media (Fig. 4).
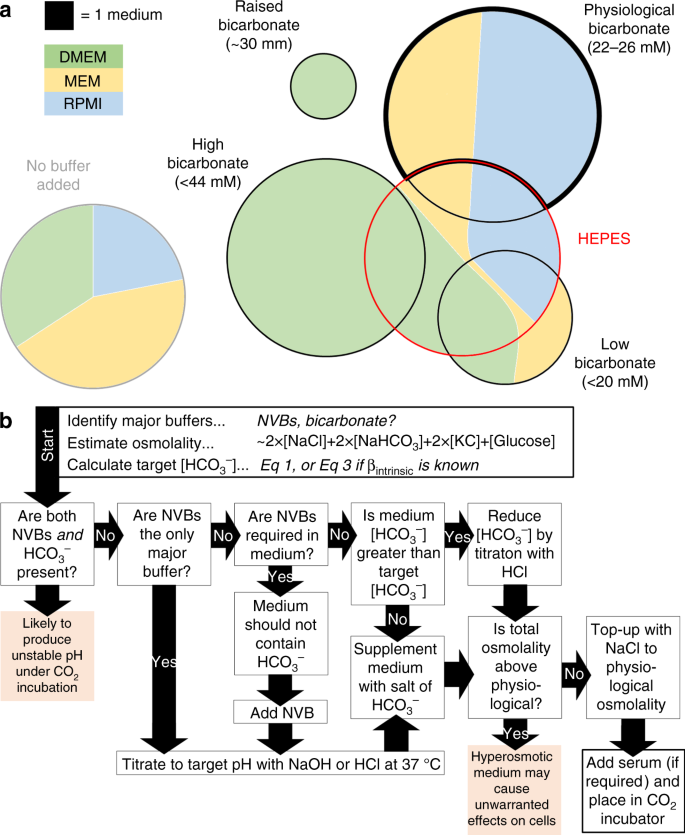
Summary of buffering regimes in commercially-available media formulations, and flow chart showing instructions for preparing media at a target pH. a Venn diagrams summarizing the commercial availability of Dulbecco's modified Eagle's medium (DMEM), minimum essential medium (MEM) or RPMI-1640 buffers (supplied by Sigma-Aldrich and Thermo Fisher Scientific), grouped by buffering regime. Surface area is proportional to the number of media available in each category. Media with physiological HCOiii − and no additional non-volatile buffer are highlighted with a thick black border. HEPES-buffered media are indicated with a ruby-red outline. b Flow chart guiding through the steps required to conform the pH of civilisation media. NVB: not-volatile buffer (eastward.one thousand. HEPES). Target [HCO3 −] for a given medium pH can exist calculated from Eq. three. Total osmolality can exist approximated equally 2 × [NaCl] + two × [NaHCOiii] + 2 × [KCl] + [Glucose]. Come across Supplementary Information 1 for farther details of these steps
In a retrospective review of articles published in Nature and Cancer Inquiry (3rd quartile of 2017, a menstruum selected at random for the purpose of this assay) reveals that only a small per centum of studies provide the necessary information about the buffering regime and pH of culture media. Three-quarters of articles published in Cancer Research and 2-thirds of life science articles published in Nature nowadays information from cultured cells. However, only under half of these articles report the manufacturer of the medium, and just a 10th requite information about the buffer composition. Only a third of all studies study the pCO2 in incubators: typically v%, although some using 10% COtwo (which then necessitates a proportional adjustment to HCO3 −). A significant number of studies utilize media containing [HCO3 −] outside the range 22–26 mM, producing a non-physiological pH. Among the studies that reported the use of 5% COtwo, approximately one-third used classical DMEM, the underlying formulation of which contains 44 mM HCOthree − (which would equilibrate to pH 7.vii in 5% CO2). Less than a 10th of studies used media supplemented with HEPES, half of which were a mixture of HEPES and bicarbonate, which are identified herein as potentially problematic (Fig. 3).
Attaining a fine degree of control over pH is realistically doable in modern culture systems, and efforts should exist made to implement the best practice in a bid to improve the accuracy, compatibility and reproducibility of measurements. The menstruum nautical chart in Fig. 6b illustrates the suggested steps in setting the pH of media. Supplementary Information one provides further details of these steps, illustrated in a selection of media from a major supplier. Based on the observations described herein, we make the following recommendations:
Recommendation 1: CO ii /HCO 3 − is the physiological buffer and therefore should be the preferred choice for biological research. Its use avoids possible unwarranted effects that exogenous buffers may have19, for example, longer-term toxicity33,34,35, Ca2+ binding (Fig. 4i) or glycolytic stimulation (Fig. 4c–f). Including CO2 as part of the buffering regime also establishes a more realistic transmembrane [COtwo] gradient for those cells that generate CO2. Additionally, the presence of HCOiii − ions activates essential membrane transport processes responsible for cellular pH homeostasis8,26. This influences steady-state intracellular pH and its sensitivity to changes in extracellular pHeastward (Fig. 5c–f), an important transduction mechanism past which medium pH modulates cellular behaviours.
Recommendation 2: media exposed to an atmosphere enriched in CO 2 must include an advisable concentration of HCO 3 − salt in club to stabilize at the required pH. COtwo/HCO3 − is unusual amongst buffers because its acidic component is a gas. Consequently, precautionary measures are warranted when treatment CO2/HCO3 −-buffered media in open chambers to avoid the loss of gas, and hence alkalinization. Whilst this chemical peculiarity is desirable in vivo because it allows the lungs to regulate buffering, it poses a claiming for experiments involving changes in ambience pCO2. Media that are to be exposed to a raised pCO2 (east.g. inside a COtwo incubator) must comprise a salt of HCO3 − in club for the buffer to promptly stabilize at a predictable pH. Conveniently, medium pH could be fix by changing the ratio of pCO2 to [HCOiii −] (Fig. 1c). When taking readings exterior COtwo incubators, it is of import to consider the pH dynamics associated with abrupt shifts in pCO2. The pH of media in modest volumes (e.g. 200 µL) volition brainstorm to rise immediately when removed from a COtwo incubator, and may require hours to attain the new equilibrium. Conversely, when preparing media for incubation, adequate time should be allowed for equilibration inside a CO2 incubator. This mitigates the gamble of an unwarranted transient alkaline stimulus imposed on cells by an out-of-equilibrium medium.
Recommendation three: media supplemented with non-volatile buffers may have unstable pH if these are prepared without consideration of the CO two -HCO three − equilibrium. For periods outside CO2 incubators, non-volatile buffers tin exist used to stabilize pH, provided that HCO3 − salts are not included (to lucifer the absence of CO2). Conversely, for experiments involving CO2 incubation, non-volatile buffers should non be used in lieu of HCO3 −, as this results in media condign more acidic than anticipated. If there is a good biological reason to supplement physiological CO2/HCO3 − with a not-volatile buffers, the medium should first be prepared with the not-volatile buffers, titrated to the target pH, and and so supplemented with a combination of COii and HCOiii − that is expected to be at equilibrium with the target pH. Some ready-made media, containing mixtures of several buffers, may not be compatible with this sequence, and thus yield unstable pH dynamics under CO2 incubation. Additionally, when preparing bespoke media with not-volatile buffers, changes in complimentary [Ca2+] and total osmolality must be considered to avert non-physiological conditions.
Recommendation 4: reporting standards must provide acceptable information near the buffering government. This should include a clarification of buffer limerick, COtwo partial pressure and, in the case of bespoke media, the steps involved in preparing media.
Methods
Cell lines and civilisation conditions
Human colorectal adenocarcinoma cells Caco2, DLD1 and NCI-H747 cells were obtained from Prof. Walter Bodmer's drove at the Weatherall Institute of Molecular Medicine (University of Oxford, United kingdom of great britain and northern ireland). Caco2 and DLD1 cells were cultivated in DMEM (Life technologies, Cat. No. 41965-039) supplemented with ten% FBS (Sigma-Aldrich) and 1% PS (100 U mL−1 penicillin, 100 µg mL−1 streptomycin; Sigma-Aldrich). NCI-H747 cells were cultivated in RPMI-1640 (Thermo Fisher Scientific, Cat. No. 21875-034), in 5% CO2 and at 37 °C. Alternatively, cells were treated with media based on NaHCOiii-free DMEM (Sigma-Aldrich, True cat. No. D7777), supplemented with various concentrations of NaHCO3, NaCl, HEPES, PIPES or MES, as indicated in effigy legends or NaHCO3 and glucose-free DMEM (Sigma-Aldrich, Cat. No. D5030) supplemented with various concentrations of glucose and NaCl, as indicated in effigy legends. Lines were authenticated past single nucleotide polymorphism (SNP)-based profiling and tested routinely for mycoplasma contamination.
Monitoring medium pH using absorbance
Medium pH was measured past PhR absorbance at 430 and 560 nm using Cytation 5 imaging plate reader (Biotek) equipped with a CO2 gas controller (Biotek). Measurements were taken from 200 µL medium in clear, flat-bottom 96-well plates (Costar) with lids at 37 °C. Media were based on NaHCO3-free DMEM (Sigma-Aldrich, Cat. No. D7777), supplemented with 10% FBS, i% PS and diverse concentrations of NaHCO3, NaCl, HEPES, PIPES or MES, as indicated in figure legends. Alternatively, media based on NaHCOthree-gratis, glucose-free DMEM (Sigma-Aldrich Cat. No. D5030) supplemented with 10% FBS, ane% PS and diverse concentrations of glucose and NaCl, as indicated in figure legends was used.
Cell growth analysis using SRB
Cells were plated in triplicates at densities of 4,000 cells per well in articulate, flat-bottom, 96-well plates with a growth area of 0.32 cm2 per well (Costar). The following 24-hour interval, the medium was replaced with 200 µL DMEM (Sigma-Aldrich, Cat. No. D7777), supplemented with ten% FBS, 1% PS and various concentrations of NaHCO3, NaCl, HEPES, PIPES or MES, equally indicated in figure legends. Alternatively, media based on NaHCO3-costless, glucose-gratis DMEM (Sigma-Aldrich Cat. No. D5030) supplemented with 10% FBS, 1% PS and various concentrations of glucose and NaCl, equally indicated in figure legends was used. Cells were cultured for vi days and pHe was monitored on each day using PhR absorbance. Later 6 days, the cells were fixed using ten% trichloroacetic acid at 4 °C for 60 min. Afterwards, they were washed with H2O 4 times, and stained with SRB (0.057% in ane% acerb acid) for 30 min. Residual SRB was removed by washing 4 times with one% acerb acid. SRB was and so dissolved in 10 mM Tris base. SRB absorbance was read at 520 nm absorbance using Cytation v imaging plate reader (Biotek).
pHi measurements
Cells were plated in triplicate at 100,000 cells per well in black wall, apartment coverslip bottom µ-plate 96-well plates with a growth surface area of 0.56 cm2 per well (Ibidi) and were left to adhere overnight. They were then incubated in media supplemented with cSNARF1-AM (5 µg mL−1, Molecular Probes) and the nuclear stain Hoechst-33342 (10 µg mL−1, Molecular Probes), for x min, then replaced with dye-gratuitous medium (twice). Images of fluorescence excited at 377 nm and collected at 447 nm (Hoechst-33342), and of fluorescence excited at 531 nm and collected at 590 nm and 640 nm (cSNARF1), were acquired using Cytation 5 imaging plate reader and its bespoke software. For media buffered with COtwo/HCOthree −, measurements were performed in an atmosphere of five% COtwo, established in the plate reader. Further analysis of the population distribution of pH data was performed with a MATLAB script (Supplementary Code 1).
Lactate and free calcium measurements
Free lactate and calcium concentrations were determined using ABX Pentra 400 (Horiba) from 150 µL aliquots of medium.
Reporting summary
Further information on experimental design is available in the Nature Inquiry Reporting Summary linked to this article.
Data availability
The datasets generating and analysed in this study are available for download as Supplementary Information ii.
References
-
Sorensen, S. P. 50. Enzymstudien. II. Mitteilung. Über die Messung und die Bedeutung der Wasserstoffionenkoncentration bei enzymatischen Prozessen. Biochem. Z. 21, 131–394 (1909).
-
Srivastava, J., Barber, D. 50. & Jacobson, K. P. Intracellular pH sensors: pattern principles and functional significance. Physiology (Bethesda) 22, 30–39 (2007).
-
Schonichen, A., Webb, B. A., Jacobson, 1000. P. & Barber, D. 50. Because protonation every bit a posttranslational modification regulating protein structure and role. Annu. Rev. Biophys. 42, 289–314 (2013).
-
White, One thousand. A. et al. Cancer-associated arginine-to-histidine mutations confer a gain in pH sensing to mutant proteins. Sci. Signal. x, https://doi.org/10.1126/scisignal.aam9931 (2017).
-
Roos, A. & Boron, W. F. Intracellular pH. Physiol. Rev. 61, 296–434 (1981).
-
Eagle, H. Buffer combinations for mammalian cell culture. Scientific discipline 174, 500–503 (1971).
-
Leem, C. H. & Vaughan-Jones, R. D. Out-of-equilibrium pH transients in the guinea-pig ventricular myocyte. J. Physiol. 509(Pt 2), 471–485 (1998).
-
Boron, Westward. F. Regulation of intracellular pH. Adv. Physiol. Educ. 28, 160–179 (2004).
-
Cost, P. J. Best practices for media selection for mammalian cells. In Vitro Jail cell Dev. Biol. Anim. 53, 673–681 (2017).
-
Coecke, S. et al. Guidance on good cell civilization practice. a report of the second ECVAM task force on good cell civilisation practice. Altern Lab. Anim. 33, 261–287 (2005).
-
Pamies, D. et al. Good jail cell civilisation practice for stem cells and stalk-cell-derived models. ALTEX 34, 95–132 (2017).
-
Pamies, D. et al. Advanced good jail cell civilization practice for human primary, stem cell-derived and organoid models as well as microphysiological systems. ALTEX 35, 353–378 (2018).
-
Geraghty, R. J. et al. Guidelines for the use of jail cell lines in biomedical research. Br. J. Cancer 111, 1021–1046 (2014).
-
Clark, Westward. M. & Lubs, H. A. The colorimetric determination of hydrogen ion concentration and its applications in bacteriology: Iii. J. Bacteriol. ii, 191–236 (1917).
-
Haas, A. R. Colorimetric conclusion of the hydrogen ion concentration in pocket-size quantities of solution. J. Biol.Chem. 38, 49 (1919).
-
Vinnakota, One thousand. C. & Kushmerick, K. J. Betoken: muscle lactate and H(+) production do have a i:1 clan in skeletal muscle. J. Appl. Physiol. (1985) 110, 1487–1489 (2011). discussion 1497.
-
Vichai, V. & Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. i, 1112–1116 (2006).
-
Eagle, H. The effect of environmental pH on the growth of normal and malignant cells. J. Jail cell. Physiol. 82, ane–viii (1973).
-
Ferguson, W. J. et al. Hydrogen ion buffers for biological inquiry. Anal. Biochem. 104, 300–310 (1980).
-
Damaghi, M. et al. Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat. Commun. 6, 8752 (2015).
-
Wilmes, A. et al. Towards optimisation of induced pluripotent cell culture: extracellular acidification results in growth arrest of iPSC prior to nutrient exhaustion. Toxicol. In Vitro 45, 445–454 (2017).
-
Erecinska, M., Deas, J. & Silver, I. A. The event of pH on glycolysis and phosphofructokinase activity in cultured cells and synaptosomes. J. Neurochem. 65, 2765–2772 (1995).
-
Hu, X., Chao, M. & Wu, H. Central role of lactate and proton in cancer cell resistance to glucose impecuniousness and its clinical translation. Bespeak. Transduct.Target Ther. ii, 16047 (2017).
-
Pedersen, S. F., Hoffmann, E. Chiliad. & Novak, I. Cell volume regulation in epithelial physiology and cancer. Front. Physiol. 4, 233 (2013).
-
Clapham, D. Eastward. Calcium signaling. Prison cell 131, 1047–1058 (2007).
-
Thomas, R. C. Cell growth factors. Bicarbonate and pHi response. Nature 337, 601 (1989).
-
Buckler, Thousand. J. & Vaughan-Jones, R. D. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-one) for intracellular pH measurement in pocket-size, isolated cells. Pflugers Curvation. 417, 234–239 (1990).
-
Thomas, J. A., Buchsbaum, R. N., Zimniak, A. & Racker, East. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18, 2210–2218 (1979).
-
Wilding, J. 50. & Bodmer, Westward. F. Cancer cell lines for drug discovery and development. Cancer Res. 74, 2377–2384 (2014).
-
Baker, M. 1,500 scientists lift the chapeau on reproducibility. Nature 533, 452–454 (2016).
-
Begley, C. M. & Ellis, L. M. Drug development: heighten standards for preclinical cancer inquiry. Nature 483, 531–533 (2012).
-
Hutchinson, L. & Kirk, R. High drug attrition rates—where are we going wrong? Nat. Rev. Clin. Oncol. viii, 189–190 (2011).
-
Hanrahan, J. Westward. & Tabcharani, J. A. Inhibition of an outwardly rectifying anion channel by HEPES and related buffers. J. Membr. Biol. 116, 65–77 (1990).
-
Lepe-Zuniga, J. L., Zigler, J. S. Jr. & Gery, I. Toxicity of light-exposed HEPES media. J. Immunol. Methods 103, 145 (1987).
-
Stea, A. & Nurse, C. A. Contrasting furnishings of HEPES vs HCO3(−)-buffered media on whole-cell currents in cultured chemoreceptors of the rat carotid trunk. Neurosci. Lett. 132, 239–242 (1991).
Acknowledgements
We thank Professor Sir Walter F. Bodmer for supplying cell lines, advice on jail cell culturing methods and for providing comments on the manuscript. Nosotros also give thanks Dr. Alzbeta Hulikova for providing comments on how to better the manuscript. P.S. wishes to give thanks Richard Vaughan-Jones for mentorship and instilling an enthusiasm for pH. The work was supported by the European Inquiry Council, SURVIVE #723997.
Author information
Affiliations
Contributions
J.M. performed the experiments, K.C.P. performed the pHi experiment and undertook the literature review, P.S. designed the enquiry and wrote the paper.
Respective author
Ethics declarations
Competing interests
The authors declare no competing interests.
Boosted information
Publisher'southward note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Admission This article is licensed under a Creative Commons Attribution iv.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, equally long as you give appropriate credit to the original author(southward) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this commodity are included in the commodity's Artistic Commons license, unless indicated otherwise in a credit line to the material. If cloth is non included in the commodity's Artistic Commons license and your intended employ is not permitted by statutory regulation or exceeds the permitted use, yous will need to obtain permission direct from the copyright holder. To view a re-create of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
Near this commodity
Cite this article
Michl, J., Park, K.C. & Swietach, P. Evidence-based guidelines for controlling pH in mammalian live-cell culture systems. Commun Biol 2, 144 (2019). https://doi.org/10.1038/s42003-019-0393-7
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/s42003-019-0393-7
Farther reading
Comments
By submitting a annotate you agree to abide past our Terms and Community Guidelines. If you find something calumniating or that does not comply with our terms or guidelines please flag information technology as inappropriate.
Source: https://www.nature.com/articles/s42003-019-0393-7
0 Response to "7. What do living systems use to control shifts in pH and why do they work?"
Post a Comment